Indications for Use: Prismira is a digital therapeutic indicated to improve attention function in adults ages 22-55 years old with primarily inattentive or combined-type Attention Deficit and Hyperactivity Disorder (ADHD). Patients who engage with Prismira demonstrate improvements in a digitally assessed measure of sustained and selective attention, Test of Variables of Attention (TOVA), and may not display benefits in typical behavioral symptoms, such as hyperactivity. Prismira should be considered for use as part of a therapeutic program that may include clinician directed therapy, medication, and/or educational programs, which further address symptoms of the disorder.
Improved Attention and Quality of Life
Results from our pivotal study with over 500 adults with ADHD
Improved Attention
Average attention functioning improved by 1.1 points — measured using the TOVA®, a validated measure of attention.
Superior Clinical Improvement
In a global measure of clinical improvement (CGI-I), assessed by blinded clinicians, there was superior clinical benefit for LumosityRx users compared to a control group.
Improved Quality of Life
Participants demonstrated a clinically meaningful mean improvement in their quality of life — 8.7 points on the Adult ADHD Quality of Life Questionnaire [AAQoL].
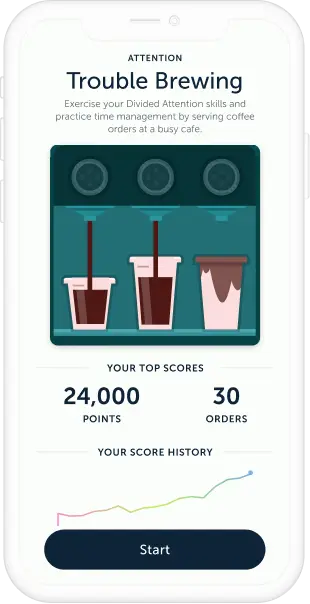
Designed to Improve Your Attention
Engaging Game-Based Experience
MOTIVATING AND IMMERSIVE
Personalized Daily Regimen
~15 MINUTES/DAY, ADAPTS TO PROGRESS
In-App Feedback
TRACK IMPROVEMENTS AND HABITS
Frequently Asked Questions
Everything you need to know about LumosityRx
Be the First to Access LumosityRx
We'll send you updates on our progress, including when LumosityRx is available.
Indications for Use
LumosityRx was cleared by the FDA under the name "Prismira."
Limitations: Prismira may not be appropriate for users with photo-sensitive epilepsy, color blindness, or physical limitations that restrict use of a mobile device. Always consult with your healthcare provider before starting any new treatment. This information does not replace professional medical advice, diagnosis, or treatment.
© 2025 LumosityRx. All rights reserved. Prescription digital therapeutic. Consult your healthcare provider.